Disclosure: This page may contain affiliate links that earn this website a small commission, at no cost to you.
Here you will find material for Experiment 5 - Preparation and Properties of Gases. This includes detailed guides for the pre-lab, data sheet, post-lab, and quiz questions. Remember, the lab may change or be modified from semester to semester!
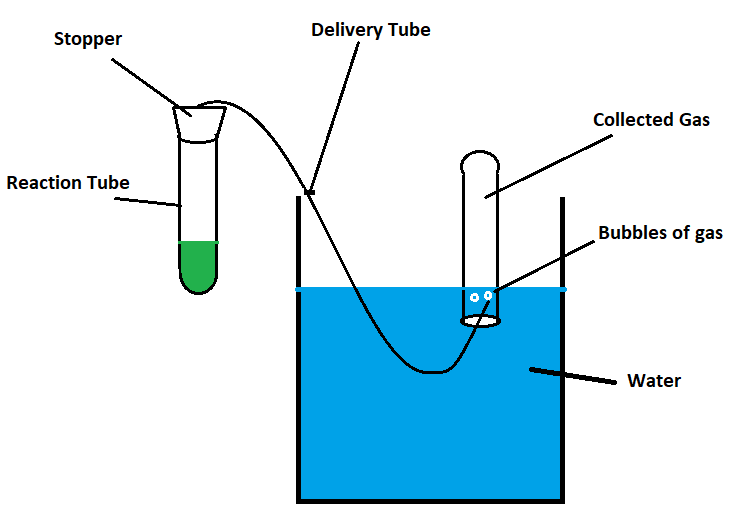
The objective of this lab is to introduce gas production, handling techniques, and common testing of gases. There will be two general methods that your will be using to produce and collect gases. Method I is done over water and is used for spontaneous reactions at room temperature. Method I is done for gases that have low solubility in water, thus the displacement of water can ensure the gas of interest is collected.
Method I
Method II will be heated and collected through displacement of air. Method II is done by displacement of air and via two types of pipets, the Z-shaped and V-shaped (see below). The Z-shaped pipet can be used for gases that are less dense than air, thus they rise and are collected in the inverted test-tube. The V-shaped pipet can be used for gases that are more dense than air, thus they sink and displace the air.
Method II (for gasses less dense than air)
Method II (for gasses denser than air)
Experiment 5 is broken up into five reactions that produce gases that you will collect and test:
Method I: Spontaneous Reactions, Done on BENCHTOP, gas collected over WATER
In 250 mL flask, put one reactant
If not done, take stopper with right-angle glass tubing in one hole and attach a length of rubber tubing to the end of the glass tubing.
Fill water trough half way with water
Fill appropriate number of 250 mL flasks full of water
Cover flask with hand and invert into trough. Repeat with remaining flasks if necessary
Place free end of rubber tubing water in the water trough
Pour second reactant into flask and cover with stopper, bubbles will emerge
Add second reactant to continue reaction
After bubbling for ~ 30 secs, Place end of tubing under mouth of one of the water filled flasks
Once flask is full of gas, seal with stopper.
Repeat with remaining flasks
- Gas 1: Carbon Dioxide | 2H+(aq) + CO32-(aq) → CO2(g) + H2O(l)
- Gas 2: Hydrogen | 2HCl(aq) +Zn(s) → H2(g) + ZnCl2(aq)
- Gas 3: Oxygen | 5H2O2(aq) + 2MnO4-(aq) + 6H+(aq) → 5O2(g) + 2Mn2+(aq) + 8H2O(l)
Method II: Heated Reactions Done in HOOD, gas collected by displacement of AIR
In 250 mL flask, place reactants
Place one-hole stopper with right-angled tubing in flask
Set stand with gauze to hold 250 mL flask
Place flask on ring stand and clamp to stablize
Clamp 2 flask vertically
Fill 1 liter beaker with 800 mL cold water
Gently heat contents of reaction flask, if bubbling to vigorously, slow by cooling in 1 liter beaker
With litmus paper, test for color change. If changes insert tubing to bottom of collection flask
With damp litmus paper at mouth of collection flask. When color changes, remove tubing, stopper original flask, and place next flask.
When both flask filled, turn off heat. After reaction flask has cooled, fill water and pour down the sink drain
- Gas 4: Hydrogen Chloride | H2SO4(aq) + NaCl(aq) → HCl(g) + Na+ + HSO4-(aq)
- Gas 5: Ammonia | 2NH4Cl(aq) + Ca(OH)2(aq) → 2NH3(g) + CaCl2(g) + 2H2O(l)
what you need
Below or a list of items you will need for lab. These items can be bought at the campus bookstore and from UH American Chemical Society (ACS). However, you may find these cheaper on sites like Amazon (links below).
CALCULATOR (LINK)
A scientific calculator is needed for calculations on quizzes. Some labs have calculations that need to be turned in before leaving.
LAB GOGGLES (LINK)
Required eye protection. Must be worn at the start of the experiment and until leaving
COMBINATION LOCK (LINK)
A combination lock is required to lock your equipment drawer for the semester.
LAB COAT (LINK)
A lab coat is required and must be worn once lab begins.
LAB MANUAL (LINK)
Specifically, General Chemistry Laboratories A Freshman Workbook. The lab manual will have all the experiment procedures you will be performing during the semester including their respective datasheet and postlab questions
what to expect
The information provided below are things you will need to know and understand to complete this experiment successfully. We have also included parts of the lab that may give you difficulties, potential bottlenecks and workarounds so you can finish the experiment efficiently. Remember, the information given by TA should have the highest importance and supersedes all information provided on this page. Your TA may present you with slides that have you doing a procedure that is different from what is given in the manual. PAY ATTENTION TO THE LECTURE!
bottlenecks
Collection of gas can be difficult. You will not be able to verify whether you have obtained the gas until you have tested it. As such, you may need to repeat the procedure in order to correctly observe each gas and its reaction. It may be helpful to split reactions using method I and those using method II. Keep in mind that doing this will require you trust your lab partners to accurately record observations.
Things to Focus on
observations
Be elaborate with your descriptions. State the obvious such as color but also the behavior. Do not just put one word description. Some TAs have been known to deduct points for descriptions not being elaborate enough. You will have to figure out how your specific TA grades.
Focus on what you observe with each gas. That is, record what you see as it is obtained via method I or method II, its color, reaction with flame, limewater and water. It is also important to understand and know the reactions for each gas that you recorded a positive result. These observations and reactions may show up on quizzes (see quiz questions).
safety
Pay attention to these following. They may show up on your quiz.
- HCl is a strong acid
- H2SO4 is a strong acid
- KMnO4 explosive with organics
- H2 flammable gas, ensure bunsen burners are off
- O2 flammable gas, ensure bunsen burners are off
- NH3 is an irritant